InVivoMAb anti-mouse ICOS
Product Details
The 7E.17G9 monoclonal antibody reacts with mouse ICOS (inducible T cell co-stimulator). ICOS is a 47-57 kDa homodimeric glycoprotein belonging to the CD28 family of costimulatory molecules. ICOS is expressed on activated T cells and upon ICOSL binding, co-stimulates T and B cell responses. The ligand Is expressed on antigen presenting cells including splenic B cells, dendritic cells, and macrophages. ICOS signaling is also thought to be important for maintaining regulatory T cell homeostasis. The 7E.17G9 antibody has been shown to block the binding of ICOSL to ICOS in vivo.Specifications
| Isotype | Rat IgG2b, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb rat IgG2b isotype control, anti-keyhole limpet hemocyanin |
| Recommended Dilution Buffer | InVivoPure pH 8.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Mouse ICOS cDNA and ICOS hexahistidine fusion protein |
| Reported Applications |
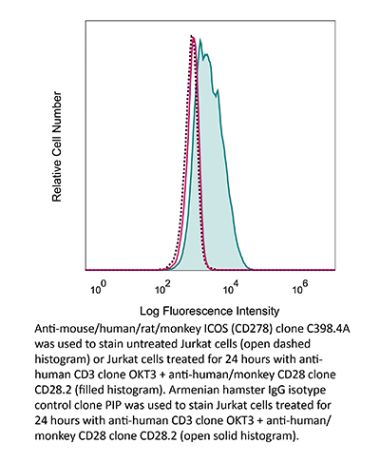
in vivo blocking of ICOS/ICOSL signaling Flow cytometry |
| Formulation |
PBS, pH 8.0 Contains no stabilizers or preservatives |
| Endotoxin |
<2EU/mg (<0.002EU/μg) Determined by LAL gel clotting assay |
| Purity |
>95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_1107622 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
Recommended Products
in vivo blocking of ICOS/ICOSL signaling, Flow Cytometry
Bauche, D., et al. (2018). "LAG3(+) Regulatory T Cells Restrain Interleukin-23-Producing CX3CR1(+) Gut-Resident Macrophages during Group 3 Innate Lymphoid Cell-Driven Colitis" Immunity 49(2): 342-352 e345. PubMed
Interleukin-22 (IL-22)-producing group 3 innate lymphoid cells (ILC3) maintains gut homeostasis but can also promote inflammatory bowel disease (IBD). The regulation of ILC3-dependent colitis remains to be elucidated. Here we show that Foxp3(+) regulatory T cells (Treg cells) prevented ILC3-mediated colitis in an IL-10-independent manner. Treg cells inhibited IL-23 and IL-1beta production from intestinal-resident CX3CR1(+) macrophages but not CD103(+) dendritic cells. Moreover, Treg cells restrained ILC3 production of IL-22 through suppression of CX3CR1(+) macrophage production of IL-23 and IL-1beta. This suppression was contact dependent and was mediated by latent activation gene-3 (LAG-3)-an immune checkpoint receptor-expressed on Treg cells. Engagement of LAG-3 on MHC class II drove profound immunosuppression of CX3CR1(+) tissue-resident macrophages. Our study reveals that the health of the intestinal mucosa is maintained by an axis driven by Treg cells communication with resident macrophages that withhold inflammatory stimuli required for ILC3 function.
in vivo blocking of ICOS/ICOSL signaling
Wang, W., et al. (2018). "RIP1 Kinase Drives Macrophage-Mediated Adaptive Immune Tolerance in Pancreatic Cancer" Cancer Cell 34(5): 757-774 e757. PubMed
Pancreatic ductal adenocarcinoma (PDA) is characterized by immune tolerance and immunotherapeutic resistance. We discovered upregulation of receptor-interacting serine/threonine protein kinase 1 (RIP1) in tumor-associated macrophages (TAMs) in PDA. To study its role in oncogenic progression, we developed a selective small-molecule RIP1 inhibitor with high in vivo exposure. Targeting RIP1 reprogrammed TAMs toward an MHCII(hi)TNFalpha(+)IFNgamma(+) immunogenic phenotype in a STAT1-dependent manner. RIP1 inhibition in TAMs resulted in cytotoxic T cell activation and T helper cell differentiation toward a mixed Th1/Th17 phenotype, leading to tumor immunity in mice and in organotypic models of human PDA. Targeting RIP1 synergized with PD1-and inducible co-stimulator-based immunotherapies. Tumor-promoting effects of RIP1 were independent of its co-association with RIP3. Collectively, our work describes RIP1 as a checkpoint kinase governing tumor immunity.
in vivo blocking of ICOS/ICOSL signaling
Liu, D., et al. (2016). "Retrogenic ICOS Expression Increases Differentiation of KLRG-1hiCD127loCD8+ T Cells during Listeria Infection and Diminishes Recall Responses" J Immunol 196(3): 1000-1012. PubMed
Following T cell encounter with Ag, multiple signals are integrated to collectively induce distinct differentiation programs within Ag-specific CD8(+) T cell populations. Several factors contribute to these cell fate decisions, including the amount and duration of Ag, exposure to inflammatory cytokines, and degree of ligation of cosignaling molecules. The ICOS is not expressed on resting T cells but is rapidly upregulated upon encounter with Ag. However, the impact of ICOS signaling on programmed differentiation is not well understood. In this study, we therefore sought to determine the role of ICOS signaling on CD8(+) T cell programmed differentiation. Through the creation of novel ICOS retrogenic Ag-specific TCR-transgenic CD8(+) T cells, we interrogated the phenotype, functionality, and recall potential of CD8(+) T cells that receive early and sustained ICOS signaling during Ag exposure. Our results reveal that these ICOS signals critically impacted cell fate decisions of Ag-specific CD8(+) T cells, resulting in increased frequencies of KLRG-1(hi)CD127(lo) cells, altered BLIMP-1, T-bet, and eomesodermin expression, and increased cytolytic capacity as compared with empty vector controls. Interestingly, however, ICOS retrogenic CD8(+) T cells also preferentially homed to nonlymphoid organs and exhibited reduced multicytokine functionality and reduced ability to mount secondary recall responses upon challenge in vivo. In sum, our results suggest that an altered differentiation program is induced following early and sustained ICOS expression, resulting in the generation of more cytolyticly potent, terminally differentiated effectors that possess limited capacity for recall response.
in vivo blocking of ICOS/ICOSL signaling
Villegas-Mendez, A., et al. (2015). "Parasite-specific CD4+IFN-gamma+IL-10+ T cells distribute within both lymphoid and non-lymphoid compartments and are controlled systemically by IL-27 and ICOS during blood-stage malaria infection" Infect Immun. pii : IAI.01100-15. PubMed
Immune-mediated pathology in IL-10 deficient mice during blood-stage malaria infection typically manifests in non-lymphoid organs, such as the liver and lung. Thus, it is critical to define the cellular sources of IL-10 in these sensitive non-lymphoid compartments during infection. Moreover, it is important to determine if IL-10 production is controlled through conserved or disparate molecular programmes in distinct anatomical locations during malaria infection, as this may enable spatiotemporal tuning of the regulatory immune response. In this study, using dual IFN-gamma-YFP and IL-10-GFP reporter mice we show that CD4+YFP+ T cells are the major source of IL-10 in both lymphoid and non-lymphoid compartments throughout the course of blood-stage P. yoelii infection. Mature splenic CD4+YFP+GFP+ T cells, which preferentially expressed high levels of CCR5, were capable of migrating to and seeding the non-lymphoid tissues, indicating that the systemically distributed host-protective cells have a common developmental history. Despite exhibiting comparable phenotypes, CD4+YFP+GFP+ T cells from the liver and lung produced significantly higher quantities of IL-10 than their splenic counterparts, showing that the CD4+YFP+GFP+ T cells exert graded functions in distinct tissue locations during infection. Unexpectedly, given the unique environmental conditions within discrete non-lymphoid and lymphoid organs, we show that IL-10 production by CD4+YFP+ T cells is controlled systemically during malaria infection through IL-27R signalling that is supported post-CD4+ T cell priming by ICOS signalling. The results in this study substantially improve our understanding of the systemic IL-10 response to malaria infection, particularly within sensitive non-lymphoid organs.
in vivo blocking of ICOS/ICOSL signaling
Krupnick, A. S., et al. (2014). "Central memory CD8+ T lymphocytes mediate lung allograft acceptance" J Clin Invest 124(3): 1130-1143. PubMed
Memory T lymphocytes are commonly viewed as a major barrier for long-term survival of organ allografts and are thought to accelerate rejection responses due to their rapid infiltration into allografts, low threshold for activation, and ability to produce inflammatory mediators. Because memory T cells are usually associated with rejection, preclinical protocols have been developed to target this population in transplant recipients. Here, using a murine model, we found that costimulatory blockade-mediated lung allograft acceptance depended on the rapid infiltration of the graft by central memory CD8+ T cells (CD44(hi)CD62L(hi)CCR7+). Chemokine receptor signaling and alloantigen recognition were required for trafficking of these memory T cells to lung allografts. Intravital 2-photon imaging revealed that CCR7 expression on CD8+ T cells was critical for formation of stable synapses with antigen-presenting cells, resulting in IFN-gamma production, which induced NO and downregulated alloimmune responses. Thus, we describe a critical role for CD8+ central memory T cells in lung allograft acceptance and highlight the need for tailored approaches for tolerance induction in the lung.
in vivo blocking of ICOS/ICOSL signaling
Rabant, M., et al. (2013). "CD40-independent help by memory CD4 T cells induces pathogenic alloantibody but does not lead to long-lasting humoral immunity" Am J Transplant 13(11): 2831-2841. PubMed
CD40/CD154 interactions are essential for productive antibody responses to T-dependent antigens. Memory CD4 T cells express accelerated helper functions and are less dependent on costimulation when compared with naive T cells. Here, we report that donor-reactive memory CD4 T cells can deliver help to CD40-deficient B cells and induce high titers of IgG alloantibodies that contribute to heart allograft rejection in CD40-/- heart recipients. While cognate interactions between memory helper T and B cells are crucial for CD40-independent help, this process is not accompanied by germinal center formation and occurs despite inducible costimulatory blockade. Consistent with the extrafollicular nature of T/B cell interactions, CD40-independent help fails to maintain stable levels of serum alloantibody and induce differentiation of long-lived plasma cells and memory B cells. In summary, our data suggest that while CD40-independent help by memory CD4 T cells is sufficient to induce high levels of pathogenic alloantibody, it does not sustain long-lasting anti-donor humoral immunity and B cell memory responses. This information may guide the future use of CD40/CD154 targeting therapies in transplant recipients containing donor-reactive memory T cells.
in vivo blocking of ICOS/ICOSL signaling, Flow Cytometry
Kadri, N., et al. (2012). "CD4(+) type II NKT cells mediate ICOS and programmed death-1-dependent regulation of type 1 diabetes" J Immunol 188(7): 3138-3149. PubMed
Type 1 diabetes (T1D) is a chronic autoimmune disease that results from T cell-mediated destruction of pancreatic beta cells. CD1d-restricted NKT lymphocytes have the ability to regulate immunity, including autoimmunity. We previously demonstrated that CD1d-restricted type II NKT cells, which carry diverse TCRs, prevented T1D in the NOD mouse model for the human disease. In this study, we show that CD4(+) 24alphabeta type II NKT cells, but not CD4/CD8 double-negative NKT cells, were sufficient to downregulate diabetogenic CD4(+) BDC2.5 NOD T cells in adoptive transfer experiments. CD4(+) 24alphabeta NKT cells exhibited a memory phenotype including high ICOS expression, increased cytokine production, and limited display of NK cell markers, compared with double-negative 24alphabeta NKT cells. Blocking of ICOS or the programmed death-1/programmed death ligand 1 pathway was shown to abolish the regulation that occurred in the pancreas draining lymph nodes. To our knowledge, these results provide for the first time cellular and molecular information on how type II CD1d-restricted NKT cells regulate T1D.
in vivo blocking of ICOS/ICOSL signaling
Charbonnier, L. M., et al. (2012). "CTLA4-Ig restores rejection of MHC class-II mismatched allografts by disabling IL-2-expanded regulatory T cells" Am J Transplant 12(9): 2313-2321. PubMed
Allograft acceptance and tolerance can be achieved by different approaches including inhibition of effector T cell responses through CD28-dependent costimulatory blockade and induction of peripheral regulatory T cells (Tregs). The observation that Tregs rely upon CD28-dependent signals for development and peripheral expansion, raises the intriguing possibility of a counterproductive consequence of CTLA4-Ig administration on tolerance induction. We have investigated the possible negative effect of CTLA4-Ig on Treg-mediated tolerance induction using a mouse model of single MHC class II-mismatched skin grafts in which long-term acceptance was achieved by short-term administration of IL-2/anti-IL-2 complex. CTLA4-Ig treatment was found to abolish Treg-dependent acceptance in this model, restoring skin allograft rejection and Th1 alloreactivity. CTLA4-Ig inhibited IL-2-driven Treg expansion, and prevented in particular the occurrence of ICOS(+) Tregs endowed with potent suppressive capacities. Restoring CD28 signaling was sufficient to counteract the deleterious effect of CTLA4-Ig on Treg expansion and functionality, in keeping with the hypothesis that costimulatory blockade inhibits Treg expansion and function by limiting the delivery of essential CD28-dependent signals. Inhibition of regulatory T cell function should therefore be taken into account when designing tolerance protocols based on costimulatory blockade.
- Immunology and Microbiology,
Transplantation elicits a clonally diverse CD8+ T cell response that is comprised of potent CD43+ effectors.
In Cell Reports on 29 August 2023 by Cohen, G. S., Kallarakal, M. A., et al.
PubMed
CD8+ T cells mediate acute rejection of allografts, which threatens the long-term survival of transplanted organs. Using MHC class I tetramers, we find that allogeneic CD8+ T cells are present at an elevated naive precursor frequency relative to other epitopes, only modestly increase in number after grafting, and maintain high T cell receptor diversity throughout the immune response. While antigen-specific effector CD8+ T cells poorly express the canonical effector marker KLRG-1, expression of the activated glycoform of CD43 defines potent effectors after transplantation. Activated CD43+ effector T cells maintain high expression of the coreceptor induced T cell costimulator (ICOS) in the presence of CTLA-4 immunoglobulin (Ig), and dual CTLA-4 Ig/anti-ICOS treatment prolongs graft survival. These data demonstrate that graft-specific CD8+ T cells have a distinct response profile relative to anti-pathogen CD8+ T cells and that CD43 and ICOS are critical surface receptors that define potent effector CD8+ T cell populations that form after transplantation. Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
- Immunology and Microbiology
ICOS immunoPET enables visualization of activated T cells and early diagnosis of murine acute gastrointestinal GvHD.
In Blood Advances on 23 August 2022 by Xiao, Z., Alam, I. S., et al.
PubMed
Allogeneic hematopoietic cell transplantation (HCT) is a well-established and potentially curative treatment for a broad range of hematological diseases, bone marrow failure states, and genetic disorders. Acute graft-versus-host disease (GvHD), mediated by donor T cells attacking host tissues, still represents a major cause of morbidity and mortality following allogeneic HCT. Current approaches to diagnosis of gastrointestinal acute GvHD rely on clinical and pathological criteria that manifest at late stages of disease. New strategies allowing for GvHD prediction and diagnosis, prior to symptom onset, are urgently needed. Noninvasive antibody-based positron emission tomography (PET) (immunoPET) imaging of T-cell activation post-allogeneic HCT is a promising strategy toward this goal. In this work, we identified inducible T-cell costimulator (ICOS) as a potential immunoPET target for imaging activated T cells during GvHD. We demonstrate that the use of the Zirconium-89-deferoxamine-ICOS monoclonal antibody PET tracer allows in vivo visualization of donor T-cell activation in target tissues, namely the intestinal tract, in a murine model of acute GvHD. Importantly, we demonstrate that the Zirconium-89-deferoxamine-ICOS monoclonal antibody PET tracer does not affect GvHD pathogenesis or the graft-versus-tumor (GvT) effect of the transplant procedure. Our data identify ICOS immunoPET as a promising strategy for early GvHD diagnosis prior to the appearance of clinical symptoms. © 2022 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
- Mus musculus (House mouse),
- Cardiovascular biology,
- Immunology and Microbiology
Overexpression of PD-1 on T cells promotes tolerance in cardiac transplantation via ICOS-dependent mechanisms.
In JCI Insight on 22 December 2021 by Borges, T. J., Murakami, N., et al.
PubMed
The programmed death 1/programmed death ligand 1 (PD-1/PD-L1) pathway is a potent inhibitory pathway involved in immune regulation and is a potential therapeutic target in transplantation. In this study, we show that overexpression of PD-1 on T cells (PD-1 Tg) promotes allograft tolerance in a fully MHC-mismatched cardiac transplant model when combined with costimulation blockade with CTLA-4-Ig. PD-1 overexpression on T cells also protected against chronic rejection in a single MHC II-mismatched cardiac transplant model, whereas the overexpression still allowed the generation of an effective immune response against an influenza A virus. Notably, Tregs from PD-1 Tg mice were required for tolerance induction and presented greater ICOS expression than those from WT mice. The survival benefit of PD-1 Tg recipients required ICOS signaling and donor PD-L1 expression. These results indicate that modulation of PD-1 expression, in combination with a costimulation blockade, is a promising therapeutic target to promote transplant tolerance.
- Cancer Research,
- Immunology and Microbiology
Molecular Imaging of Chimeric Antigen Receptor T Cells by ICOS-ImmunoPET.
In Clinical Cancer Research on 15 February 2021 by Simonetta, F., Alam, I. S., et al.
PubMed
Immunomonitoring of chimeric antigen receptor (CAR) T cells relies primarily on their quantification in the peripheral blood, which inadequately quantifies their biodistribution and activation status in the tissues. Noninvasive molecular imaging of CAR T cells by PET is a promising approach with the ability to provide spatial, temporal, and functional information. Reported strategies rely on the incorporation of reporter transgenes or ex vivo biolabeling, significantly limiting the application of CAR T-cell molecular imaging. In this study, we assessed the ability of antibody-based PET (immunoPET) to noninvasively visualize CAR T cells. After analyzing human CAR T cells in vitro and ex vivo from patient samples to identify candidate targets for immunoPET, we employed a syngeneic, orthotopic murine tumor model of lymphoma to assess the feasibility of in vivo tracking of CAR T cells by immunoPET using the 89Zr-DFO-anti-ICOS tracer, which we have previously reported. Analysis of human CD19-CAR T cells during activation identified the Inducible T-cell COStimulator (ICOS) as a potential target for immunoPET. In a preclinical tumor model, 89Zr-DFO-ICOS mAb PET-CT imaging detected significantly higher signal in specific bone marrow-containing skeletal sites of CAR T-cell-treated mice compared with controls. Importantly, administration of ICOS-targeting antibodies at tracer doses did not interfere with CAR T-cell persistence and function. This study highlights the potential of ICOS-immunoPET imaging for monitoring of CAR T-cell therapy, a strategy readily applicable to both commercially available and investigational CAR T cells.See related commentary by Volpe et al., p. 911. ©2020 American Association for Cancer Research.
- Mus musculus (House mouse),
- Immunology and Microbiology
Host immunology and rational immunotherapy for carbapenem-resistant Klebsiella pneumoniae infection.
In JCI Insight on 23 April 2020 by Iwanaga, N., Sandquist, I., et al.
PubMed
Infections due to carbapenem-resistant Klebsiella pneumoniae have emerged as a global threat due to its widespread antimicrobial resistance. Transplant recipients and patients with hematologic malignancies have high mortality rate, suggesting host factors in susceptibility. We developed a model of pulmonary infection using ST258 strain C4, KPC-2 clone, which are predominant K. pneumoniae carbapenemase-producing (KPC-producing) bacteria, and demonstrated that Rag2-/- Il2rg-/- mice - but not WT C57BL/6 or Rag2-/- mice - were susceptible to this opportunistic infection. Using single cell RNA sequencing in infected Rag2-/- mice, we identified distinct clusters of Ifng+ NK cells and Il17a+, Il22+, and inducible T cell costimulatory molecule-positive (ICOS+) group 3 innate lymphoid cells (ILCs) that were critical for host resistance. As solid organ transplantation is a risk factor, we generated a more clinically relevant model using FK506 in WT C57BL/6 mice. We further demonstrated that immunotherapy with recombinant IL-22 treatment ameliorated the ST258 pulmonary infection in both FK506-treated WT mice and Rag2-/- Il2rg-/- mice via hepatic IL-22ra1 signaling. These data support the development of host-directed immunotherapy as an adjunct treatment to new antibiotics.
- Endocrinology and Physiology,
- Immunology and Microbiology
Tolerogenic Dendritic Cells Attenuate Experimental Autoimmune Antimyeloperoxidase Glomerulonephritis.
In Journal of the American Society of Nephrology : JASN on 1 November 2019 by Odobasic, D., Oudin, V., et al.
PubMed
Background Because of their capacity to induce antigen-specific immunosuppression, tolerogenic dendritic cells are a promising tool for treatment of autoimmune conditions, such as GN caused by autoimmunity against myeloperoxidase (MPO). We sought to generate tolerogenic dendritic cells to suppress anti-MPO GN by culturing bone marrow cells with an NFκB inhibitor (BAY 11-7082) and exposing them to a pulse of MPO. After administering these MPO/BAY dendritic cells or saline to mice with established anti-MPO or anti-methylated BSA (mBSA) immunity, we assessed immune responses and GN. We also examined mechanisms of action of MPO/BAY dendritic cells. MPO/BAY dendritic cells decreased anti-MPO immunity and GN without inhibiting immune responses against mBSA; they also induced IL-10-producing regulatory T cells in MPO-immunized mice without affecting IL-10+ CD4+Foxp3- type 1 regulatory T cells or regulatory B cells. MPO/BAY dendritic cells did not inhibit anti-MPO immunity when CD4+Foxp3+ cells were depleted in vivo, showing that regulatory T cells are required for their effects. Coculture experiments with dendritic cells and CD4+Foxp3- or CD4+Foxp3+ cells showed that MPO/BAY dendritic cells generate Foxp3+ regulatory T cells from CD4+Foxp3- cells through several pathways, and induce IL-10+ regulatory T cells via inducible costimulator (ICOS), which was confirmed in vivo. Transfer of MPO/BAY dendritic cell-induced regulatory T cells in vivo, with or without anti-IL-10 receptor antibody, demonstrated that they suppress anti-MPO immunity and GN via IL-10. MPO/BAY dendritic cells attenuate established anti-MPO autoimmunity and GN in an antigen-specific manner through ICOS-dependent induction of IL-10-expressing regulatory T cells. This suggests that autoantigen-loaded tolerogenic dendritic cells may represent a novel antigen-specific therapeutic option for anti-MPO GN. Copyright © 2019 by the American Society of Nephrology.
- Cancer Research,
- Immunology and Microbiology
RIP1 Kinase Drives Macrophage-Mediated Adaptive Immune Tolerance in Pancreatic Cancer.
In Cancer Cell on 12 November 2018 by Wang, W., Marinis, J. M., et al.
PubMed
Pancreatic ductal adenocarcinoma (PDA) is characterized by immune tolerance and immunotherapeutic resistance. We discovered upregulation of receptor-interacting serine/threonine protein kinase 1 (RIP1) in tumor-associated macrophages (TAMs) in PDA. To study its role in oncogenic progression, we developed a selective small-molecule RIP1 inhibitor with high in vivo exposure. Targeting RIP1 reprogrammed TAMs toward an MHCIIhiTNFα+IFNγ+ immunogenic phenotype in a STAT1-dependent manner. RIP1 inhibition in TAMs resulted in cytotoxic T cell activation and T helper cell differentiation toward a mixed Th1/Th17 phenotype, leading to tumor immunity in mice and in organotypic models of human PDA. Targeting RIP1 synergized with PD1-and inducible co-stimulator-based immunotherapies. Tumor-promoting effects of RIP1 were independent of its co-association with RIP3. Collectively, our work describes RIP1 as a checkpoint kinase governing tumor immunity. Copyright © 2018 Elsevier Inc. All rights reserved.
- Mus musculus (House mouse),
- Immunology and Microbiology
LAG3+ Regulatory T Cells Restrain Interleukin-23-Producing CX3CR1+ Gut-Resident Macrophages during Group 3 Innate Lymphoid Cell-Driven Colitis.
In Immunity on 21 August 2018 by Bauche, D., Joyce-Shaikh, B., et al.
PubMed
Interleukin-22 (IL-22)-producing group 3 innate lymphoid cells (ILC3) maintains gut homeostasis but can also promote inflammatory bowel disease (IBD). The regulation of ILC3-dependent colitis remains to be elucidated. Here we show that Foxp3+ regulatory T cells (Treg cells) prevented ILC3-mediated colitis in an IL-10-independent manner. Treg cells inhibited IL-23 and IL-1β production from intestinal-resident CX3CR1+ macrophages but not CD103+ dendritic cells. Moreover, Treg cells restrained ILC3 production of IL-22 through suppression of CX3CR1+ macrophage production of IL-23 and IL-1β. This suppression was contact dependent and was mediated by latent activation gene-3 (LAG-3)-an immune checkpoint receptor-expressed on Treg cells. Engagement of LAG-3 on MHC class II drove profound immunosuppression of CX3CR1+ tissue-resident macrophages. Our study reveals that the health of the intestinal mucosa is maintained by an axis driven by Treg cells communication with resident macrophages that withhold inflammatory stimuli required for ILC3 function. Copyright © 2018 Elsevier Inc. All rights reserved.
- Immunology and Microbiology,
- Mus musculus (House mouse)
Extrafollicular CD4+ T-B interactions are sufficient for inducing autoimmune-like chronic graft-versus-host disease.
In Nature Communications on 17 October 2017 by Deng, R., Hurtz, C., et al.
PubMed
Chronic graft-versus-host disease (cGVHD) is an autoimmune-like syndrome mediated by pathogenic CD4+ T and B cells, but the function of extrafollicular and germinal center CD4+ T and B interactions in cGVHD pathogenesis remains largely unknown. Here we show that extrafollicular CD4+ T and B interactions are sufficient for inducing cGVHD, while germinal center formation is dispensable. The pathogenesis of cGVHD is associated with the expansion of extrafollicular CD44hiCD62loPSGL-1loCD4+ (PSGL-1loCD4+) T cells. These cells express high levels of ICOS, and the blockade of ICOS/ICOSL interaction prevents their expansion and ameliorates cGVHD. Expansion of PSGL-1loCD4+ T cells is also prevented by BCL6 or Stat3 deficiency in donor CD4+ T cells, with the induction of cGVHD ameliorated by BCL6 deficiency and completely suppressed by Stat3 deficiency in donor CD4+ T cells. These results support that Stat3- and BCL6-dependent extrafollicular CD4+ T and B interactions play critical functions in the pathogenesis of cGVHD.Chronic graft-versus-host disease (cGVHD) is mediated by specific CD4 and B cells, but the relative contribution of extrafollicular and germinal centre (GC) T-B interaction is unclear. Here the authors show that the extrafollicular expansion of a specific CD4 T subset is sufficient for inducing cGVHD while GC is dispensable.
- Immunology and Microbiology,
- Neuroscience
CD40-independent help by memory CD4 T cells induces pathogenic alloantibody but does not lead to long-lasting humoral immunity.
In American Journal of Transplantation : Official Journal of the American Society of Transplantation and the American Society of Transplant Surgeons on 1 November 2013 by Rabant, M., Gorbacheva, V., et al.
PubMed
CD40/CD154 interactions are essential for productive antibody responses to T-dependent antigens. Memory CD4 T cells express accelerated helper functions and are less dependent on costimulation when compared with naïve T cells. Here, we report that donor-reactive memory CD4 T cells can deliver help to CD40-deficient B cells and induce high titers of IgG alloantibodies that contribute to heart allograft rejection in CD40-/- heart recipients. While cognate interactions between memory helper T and B cells are crucial for CD40-independent help, this process is not accompanied by germinal center formation and occurs despite inducible costimulatory blockade. Consistent with the extrafollicular nature of T/B cell interactions, CD40-independent help fails to maintain stable levels of serum alloantibody and induce differentiation of long-lived plasma cells and memory B cells. In summary, our data suggest that while CD40-independent help by memory CD4 T cells is sufficient to induce high levels of pathogenic alloantibody, it does not sustain long-lasting anti-donor humoral immunity and B cell memory responses. This information may guide the future use of CD40/CD154 targeting therapies in transplant recipients containing donor-reactive memory T cells. © Copyright 2013 The American Society of Transplantation and the American Society of Transplant Surgeons.